New Drugs For Cancer Treatment Approved By FDA
In May of this year, the US Food and Drug Administration (FDA) approved new drugs for cancer indications. These drugs are tarlatamab-dlle (Imdelltra), lisocabtagene maraleucel (Breyanzi), and selpercatinib (Retevmo). These drugs are aimed at the treatment of different types of cancers such as lymphoma, melanoma and non-small cell lung cancer. Below are the details on each of the approved drugs.
Tarlatamab-dlle (Imdelltra)
Tarlatamab-dlle (Imdelltra) is a first-time approved drug for the treatment of adult patients with relapsed or refractory mantle cell lymphoma. The drug, which was developed by Innate Pharma, works by targeting and killing malignant lymphocytes in the body. Human trials have shown that the drug is effective in treating the disease, with a high objective response rate recorded in patients who had undergone previous treatments.
Lisocabtagene maraleucel (Breyanzi)
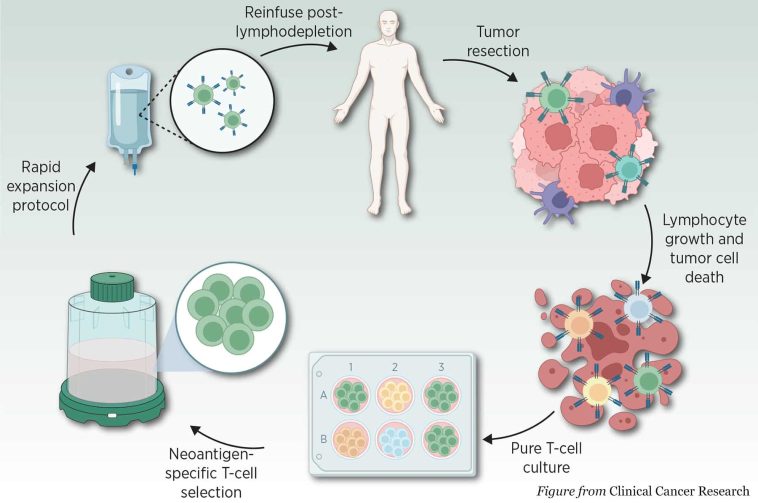
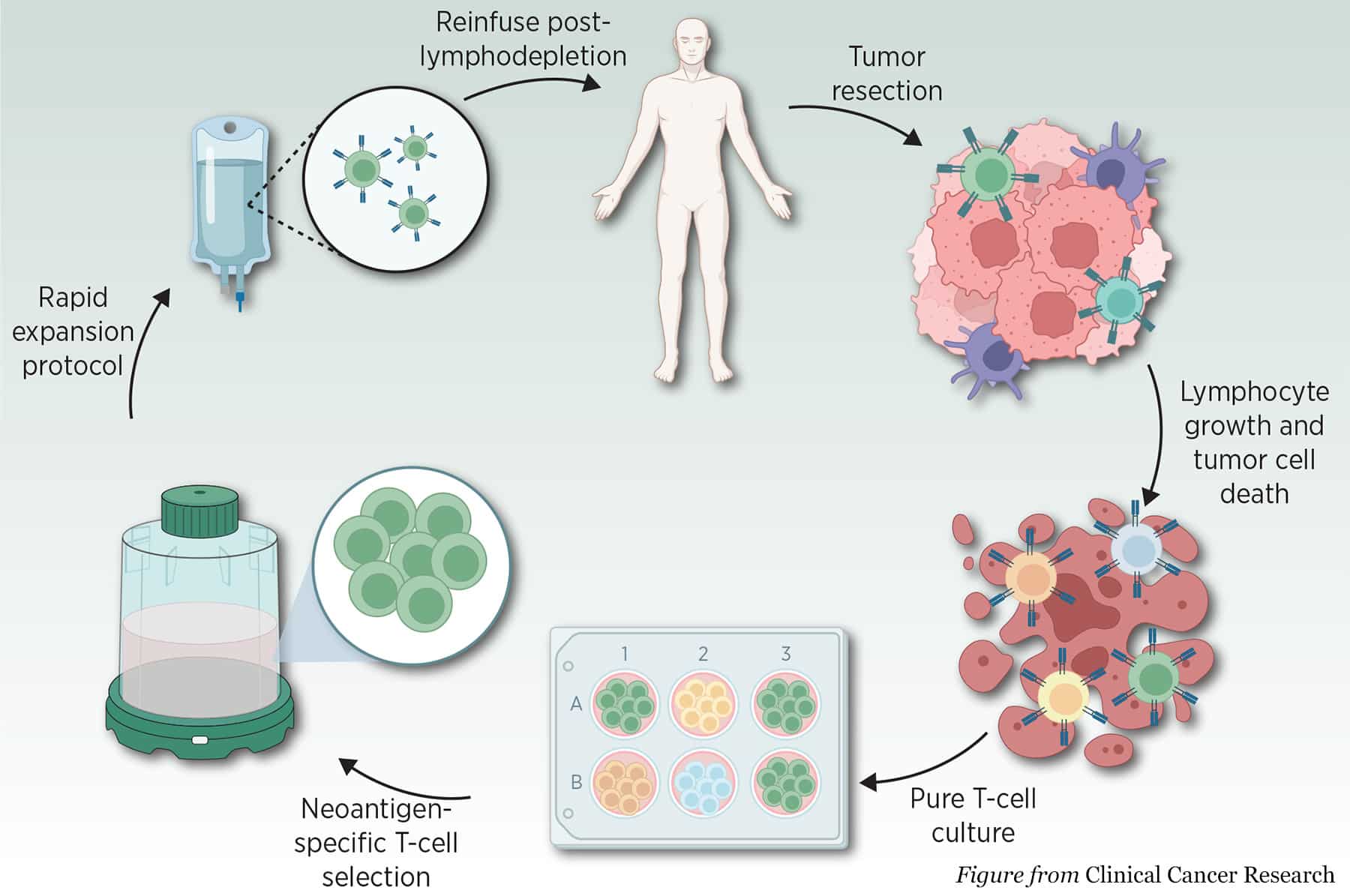
Lisocabtagene maraleucel (Breyanzi) is a drug created by Bristol-Myers Squibb. It was approved for two new indications, the treatment of adult patients with relapsed or refractory large B-cell lymphoma and high-grade B-cell lymphoma. The drug is an immunotherapy that works by genetically modifying a patient’s T-cells to target and kill cancer cells within the body. According to clinical trials, the drug has demonstrated a high response rate, leading to complete remissions in patients who were not responding to previous therapies.
Selpercatinib (Retevmo)
Selpercatinib (Retevmo) is a drug developed by Eli Lilly and Company. It was approved by the FDA to treat patients with non-small cell lung cancer, RET-fusion-positive. The drug works by selectively inhibiting mutated protein receptors in patients whose tumors express RET gene fusions. According to clinical trials, the drug led to significant tumor response rates in patients who have already undergone chemotherapy and other treatments.
Conclusion
The development of new drugs for cancer treatment has shown promise in recent years and the approval of these new treatments by the FDA is a significant development. These drugs offer new hope to patients who have not responded to traditional treatments and provide new options for treating different types of cancer. The future looks bright for cancer patients, with researchers continuing to discover new ways of treating and fighting this debilitating disease.
Originally Post From https://www.cancertherapyadvisor.com/features/may-2024-oncology-drug-approvals/
Read more about this topic at
Cancer drug approvals and setbacks in 2023
Cancer Drug Approvals That Displaced Existing Standard …