Tirzepatide: A Promising Treatment Option for Obstructive Sleep Apnea in Patients with Obesity
Introduction
The prevalence of obstructive sleep apnea (OSA) is increasing worldwide, affecting over 900 million people globally. OSA is a condition that is marked by the repetitive pharyngeal collapse during sleep, leading to apneas, hypopneas, hypoxemia, hypercapnia, and arousal, with significant symptoms like excessive daytime sleepiness and an increased cardiovascular risk. OSA affects 40% of people who suffer from moderate-to-severe OSA. Traditional treatments for the condition focus on mechanical support, primarily positive airway pressure (PAP) therapy, which improves the apnea-hypopnea index (AHI) but has limited adherence and no proven reduction in cardiovascular outcomes. Alternative treatments like mandibular advancement and upper-airway surgery are either less effective or invasive. Moreover, OSA has no approved pharmaceuticals, making weight management crucial.
Research Study
In a recent study published in the New England Journal of Medicine, researchers investigated the effects of tirzepatide on adults with obesity and moderate-to-severe obstructive sleep apnea (OSA). Tirzepatide, a long-acting GIP (short for glucose-dependent insulinotropic polypeptide) and GLP-1 (short for glucagon-like peptide-1) receptor agonist, has shown a significant decrease in body weight, blood pressure, and inflammation, suggesting that it could be effective for OSA by addressing obesity and its related complications.
The SURMOUNT-OSA Trials
The researchers presented the results of two SURMOUNT-OSA phase III trials, which investigated the safety and efficacy of tirzepatide in treating adults with OSA and obesity. The SURMOUNT-OSA trials consisted of two phase III, double-blinded, randomized controlled studies conducted between June 2022 and March 2024 across 60 sites in nine countries, aimed at evaluating the safety and efficacy of tirzepatide in adults with moderate-to-severe OSA (AHI ≥15 events per hour) and obesity (BMI ≥30 or ≥27 in Japan).
Trial 1 included participants not able or not willing to use PAP therapy, while trial two involved participants using PAP therapy for at least three months. Participants with type 1 or 2 diabetes, recent significant weight change, planned surgery for sleep apnea or obesity, major craniofacial abnormalities, and central or mixed sleep apnea were excluded. After screening for four weeks, participants were assigned to trial 1 (n = 234) or trial 2 (n = 235) and randomly given tirzepatide or a placebo once weekly for 52 weeks.
Results and Analysis
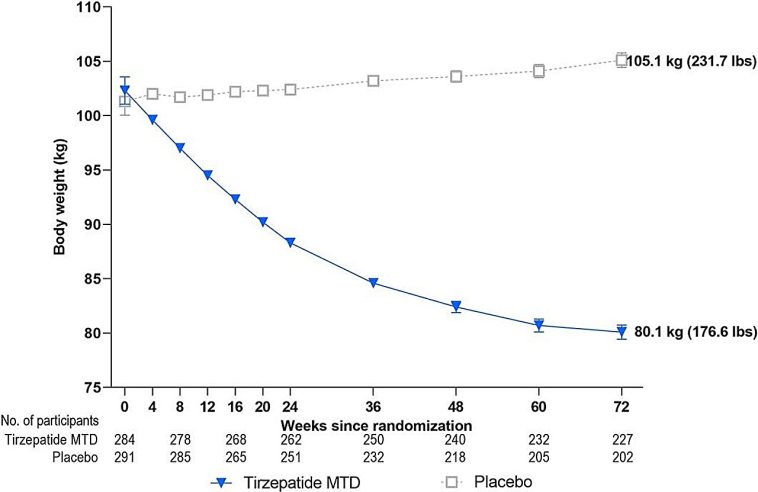
The primary endpoint was the alteration in AHI from baseline. Secondary endpoints included percent change in AHI, reduction in AHI by at least 50%, achieving AHI of > five events per hour, change in body weight, high-sensitivity C-reactive protein (hsCRP) concentration, hypoxic burden, patient-reported outcomes measurement information system (PROMIS) scores for sleep disturbance (PROMIS-SD) and sleep-related impairment (SRI), and systolic blood pressure (SBP). Adverse and serious adverse events were monitored throughout the reporting period.
Estimand analysis showed that tirzepatide treatment significantly reduced the AHI as compared to the placebo in both trials (p<0.001). Additionally, participants on tirzepatide showed improvements in sleep apnea-specific hypoxic burden, and more than 50% achieved clinically meaningful reductions in the AHI. Further, participants who received tirzepatide in both trials 1 and 2 experienced significant reductions in PROMIS-SRI and PROMIS-SD T scores as well as body weight, systolic blood pressure, and hsCRP concentration. No deaths were reported during the trials. However, participants receiving tirzepatide reported higher rates of gastrointestinal adverse events, with two cases of acute pancreatitis in trial 2.
Conclusion
In conclusion, the two trials showed that tirzepatide was found to bring about a clinically significant improvement in sleep-disordered breathing, sleep disturbance, and sleep-related impairment while reducing OSA-related cardiovascular risk factors, including AHI, body weight, hypoxic burden, hsCRP concentration, and SBP in individuals with moderate-to-severe OSA and obesity. These results highlight tirzepatide’s potential as a treatment option for improving sleep-related outcomes in this population.
Further studies are needed to investigate the long-term efficacy and safety of tirzepatide in OSA patients with and without obesity.
Originally Post From https://www.news-medical.net/news/20240626/New-drug-tirzepatide-significantly-improves-sleep-apnea-and-weight-loss.aspx
Read more about this topic at
Use of Tirzepatide Shown to Improve Sleep Apnea and …
Study Identifies First Drug Therapy for Sleep Apnea